Thermite Reaction: A 125-Year-Old Reaction That Keeps Our World Moving
Share

Introduction
The thermite reaction is a chemical marvel that was discovered over 125 years ago and continues to play an essential role in modern technology. Unlike conventional explosives, the thermite reaction produces a controlled release of immense heat, making it perfect for welding, metal purification, and even some unexpected industrial applications. In this article, we’ll dive into what makes the thermite reaction so special, its history, and how it’s used today.
What is the Thermite Reaction?
The thermite reaction involves the combination of metal oxides with aluminum powder, resulting in an extremely exothermic process. The reaction releases temperatures exceeding 2,500°C, which is more than enough to melt metals like iron, copper, or chromium.
This chemical reaction, discovered by Hans Goldschmidt in the late 1800s, was originally developed to produce pure metals. The energy output of the thermite reaction made it ideal for various industrial applications, leading to its extensive use in areas like rail welding and even weapon demilitarization.

The History of the Thermite Reaction
Hans Goldschmidt and his brother Carl, who managed their family’s chemical factory, were experimenting with ways to produce pure metals for making vibrant dyes. In a time when most dyes were faint, their search for pure metals was driven by a desire to make brighter and more durable colors. During this experimentation, Hans discovered that reacting metal oxides with aluminum powder could efficiently produce pure metals, and thus the thermite reaction was born.
Goldschmidt patented the thermite process in 1895, noting its simplicity and extraordinary effects. Since then, the reaction has found a range of practical uses, from welding train tracks to aiding in remote repairs that require high temperatures.
How the Thermite Reaction Works
The thermite reaction is driven by the affinity of aluminum for oxygen, which forms very strong bonds. When the aluminum reacts with metal oxides, it replaces the existing metal, forming aluminum oxide and pure metal in the process. The heat generated in this exothermic reaction can exceed 2,500°C, enough to melt most metals.
One interesting feature of the thermite reaction is that it requires a high ignition temperature to get started—often achieved with barium hydroxide igniters or similar compounds. Once started, however, the reaction is unstoppable, continuing until all reactants are consumed.

Thermite Reaction in Action
The thermite reaction is not just a chemical curiosity; it has practical, real-world uses:
- Rail Welding: One of the most famous uses of the thermite reaction is in welding railroad tracks. Given the difficulty of transporting welding equipment to remote rail locations, thermite is an ideal solution. It can be set up quickly and easily, requiring only two technicians and basic tools. Thermite welding has ensured that millions of train passengers experience smooth rides.
- Weapon Demilitarization: The reaction’s intense heat has also been used to destroy weapons. After the Cold War, thermite was employed to weld shut tank barrels and destroy other old military equipment, rendering them unusable.
- Data Destruction: A more modern application of the thermite reaction is in data destruction. Thermite can be used to generate enough heat to melt the platters of hard drives, ensuring that sensitive information becomes unrecoverable. This controlled application of thermite heat has proven useful in cybersecurity contexts.
The Unique Properties of Thermite
Unlike explosives, the thermite reaction involves both solid and liquid products, which means there is no rapid expansion of gases, and hence, no explosion. Instead, the power of thermite lies in its controlled release of energy.
The aluminum powder used in the reaction is coated with aluminum oxide, which acts as a stabilizer. This prevents spontaneous ignition and allows for a high level of control, ensuring the thermite reaction only starts when deliberately ignited.
Seeing Thermite Up Close
One of the most impressive demonstrations of the thermite reaction involved filming the reaction from inside a specially crafted crucible with thermal-resistant glass. This setup allowed scientists to see the inner workings of the reaction—molten metal pulsing and expanding outward in bursts. The bright orange glow and the intense heat of the reaction are similar to looking directly at the sun, making it one of the most visually spectacular chemical reactions ever recorded.
Safety and Stability
Handling thermite might sound dangerous, but surprisingly, thermite mixtures are quite stable. Even if exposed to a propane torch, it remains unreactive until a sufficiently high ignition temperature is reached. This stability makes thermite safe to transport and store, provided that proper precautions are taken during ignition.
Conclusion
The thermite reaction is more than just a spectacular display of heat and molten metal. It has served practical purposes in industries like welding and weapon destruction, all while providing scientists with an example of how intense heat can be harnessed in a controlled manner. Whether it’s helping to weld railroad tracks or destroying outdated weapons, the thermite reaction is a remarkable process that continues to play an important role in our world.
From its discovery by Hans Goldschmidt to its modern uses, the thermite reaction remains a powerful example of the potential of chemical reactions to shape our infrastructure and technology. So next time you see a train gliding smoothly on its tracks, remember—the power of thermite helped make that possible.
- Braised Halibut with Carrots and Coriander: A Weeknight Delight
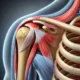
- Understanding Supraspinatus Tendinopathy with Partial Thickness Tear: Symptoms, Treatment, and Recovery
- Stevia: Why You Should Always Read Labels on Keto-Friendly Products Carefully
- Erythritol: A Comprehensive Guide to the Pros and Cons of This Popular Sweetener
- Tasty- Yummy Tex-Mex Chicken and Rice